SciNote
4.2
198
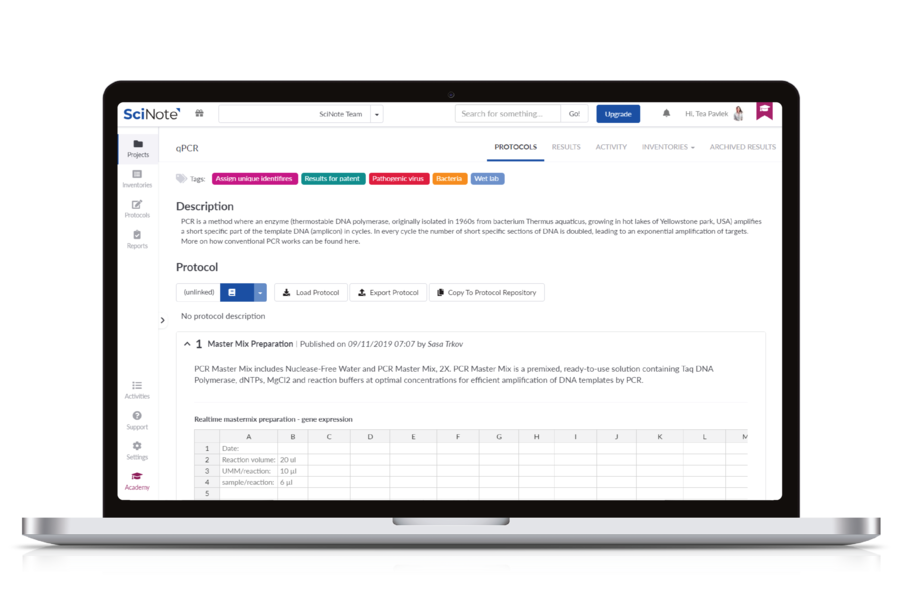
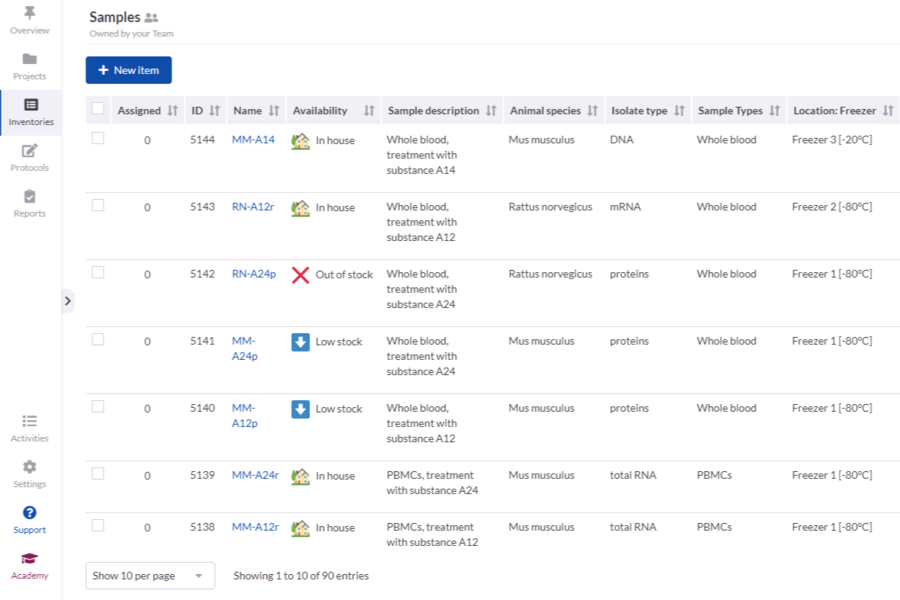
SciNote is a cloud-based electronic lab notebook (ELN) with built-in inventory, compliance, and team management tools. It is trusted by various organizations and scientists worldwide. Each premium plan comes with top-rated customer support and a customer success manager. SciNote provides data management functionalities such as inventory tracking, protocol and SOP management, compliance, team management and collaboration, integrations and API, project management, and safety and security of data. SciNote is based in Middleton, WI, USA, with offices in Europe.
Strengths
-
User-friendly interface
Easy to navigate and use
-
Cloud-based storage
Accessible from anywhere with internet connection
-
Customizable workflows
Tailored to specific research needs
Weaknesses
-
Limited integrations
Not compatible with all software
-
Limited collaboration features
Not ideal for large teams
-
Limited reporting options
May not meet all reporting needs
Opportunities
- Increasing market potential
- Expanding functionality
- Expanding user base
Threats
- May struggle to gain market share
- Sensitive data may be at risk
- May not meet all regulatory requirements
Ask anything of SciNote with Workflos AI Assistant
https://scinote.net/
Apolo
Squeak squeak, I'm a cute squirrel working for Workflos and selling software.
I have extensive knowledge of our software products and am committed to
providing excellent customer service.
What are the pros and cons of the current application?
How are users evaluating the current application?
How secure is the current application?
Media
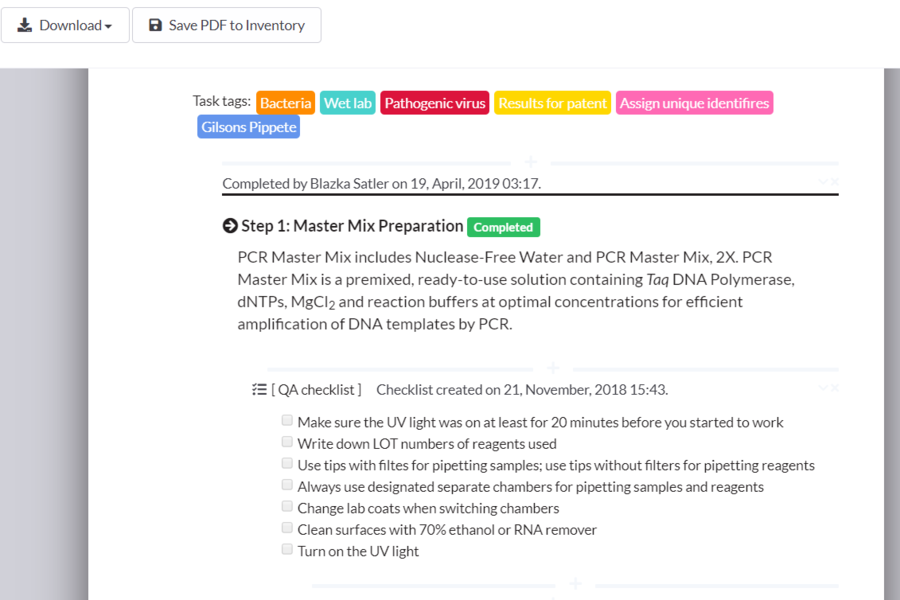
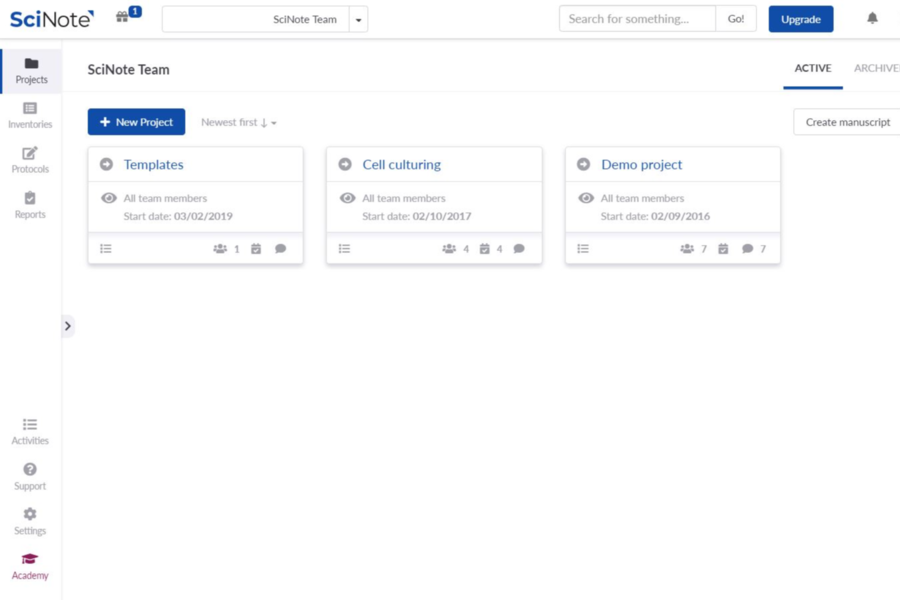
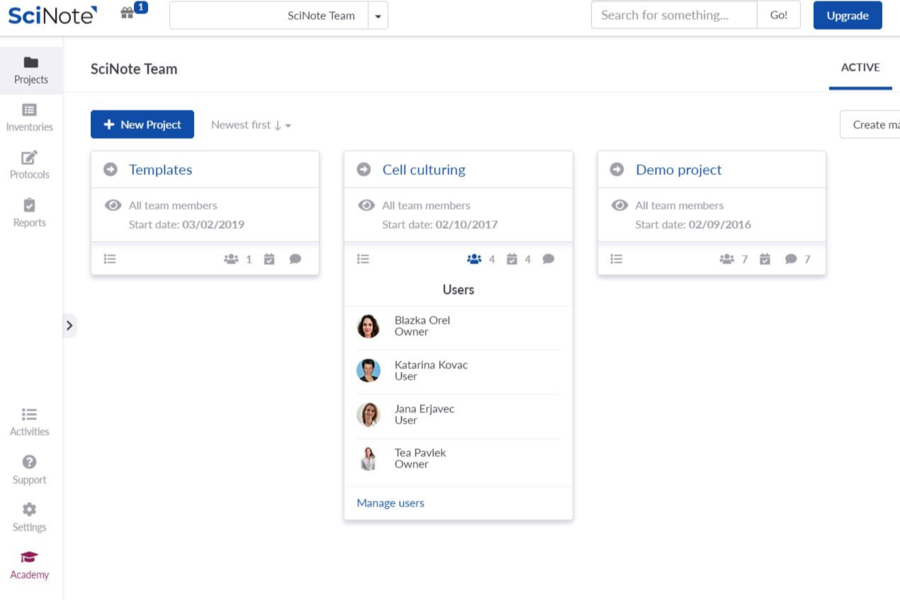


















SciNote Plan
SciNote offers a free version with basic features and paid plans starting at $19/month with additional features and user licenses.
Essential
Free Trial
All the features you need to manage your lab digitally
Team management & collaboration
Unlimited inventory items
Unlimited notes & notebooks
Customizable experiment templates
Top-rated customer support & onboarding
Microsoft Office integration
Automated reporting
Validated
Free Trial
Meet electronic record compliance, such as FDA 21 CFR part 11
Everything in the Essential Plan, PLUS
Cross-team collaboration
Electronic signatures and audit trails
Signature notifications
Task locking
Platinum
Free Trial
Maintain quality assurance in a GxP environment
Everything in the Validated Plan, PLUS
Installation Qualification (IQ)
Operational Qualification (OQ)
Test instance
Controlled releases
Priority support
Academia
Free Trial
Manage your research and your lab
Customizable experiment templates
Collaboration & task management
Unlimited notes & notebooks
Unlimited inventory items
Microsoft Office integration
Automated reporting
Top-rated customer support & onboarding